Abstract
Introduction
Poor Graft Function (PGF) characterized by 2 or more lineage cytopenias in the setting of complete donor chimerism is a recognized complication of allogeneic stem cell transplantation (alloSCT). Recurrent CMV viremia jeopardizes blood count recovery in PGF through direct effect of the virus on bone marrow function as well as myelosuppression/organ toxicity due to widely available antivirals such as val/ganciclovir or foscarnet.
Methods
To assess the clinical effect and resource utilization of recurrent CMV viremia on patients with PGF we performed a retrospective analysis of alloSCT recipients at our Centre from 2018-2019. Patients were classified as having PGF based on the following parameters: 1) Donor myeloid chimerism ≥95% at last assessment, 2) 2 or more lineage cytopenias defined by Hb ≤85g/L, Neutrophils ≤1.0x10 9/L, Platelets ≤60x10 9/L, 3) cytopenias present for 10 days after D30. CMV viral loads were monitored by q PCR twice weekly with reactivation defined as the first reading quantifiable above the threshold of detection of the assay. Recurrent CMV reactivation was defined as detectable CMV after an interval of 4weeks without detectable virus, with patients who reactivated within 4 weeks of an undetectable viral load defined as prolonged persisting infection. Patients who died before D60 or who relapsed within the 1 st 100 days or those who had a prior alloSCT were excluded. Baseline factors in the CMV seropositive PGF group were evaluated by Chi Squared and Kruskall-Wallis test for categorical and continuous variables respectively to establish any associations with recurrent or persisting CMV. Survival analysis of PGF patients was performed by the Kaplan Meier method with patients stratified by the following: Recurrent CMV/Prolonged persisting infection (RP-CMV), Single CMV reactivation (S-CMV), No CMV reactivations (N-CMV) and CMV sero-negativity (S-NCMV). A descriptive analysis of the number of hospital admissions in addition to the initial alloSCT admission (extra admissions) and total hospital days was also performed.
Results
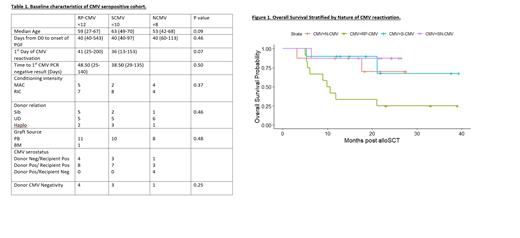
There were 155 eligible patients of which 38 (24%) fulfilled criteria for PGF. The median follow-up of the cohort was 26 months (95%CI:23.1-28). The median overall survival (OS) of the cohort was not reached with an estimated 2 year OS of 73%. The 2 year OS of PGF patients was 59% compared to 78% in the non PGF group. There were no significant baseline associations found with RP-CMV (Table 1). Figure 1 demonstrates the survival of patients by nature of CMV reactivation. The 2 year OS by CMV reactivation was as follows: RP-CMV 25%, S-CMV 68%, N-CMV 70%, SN-CMV 87%.
On univariate analysis, RP-CMV was significantly associated with mortality [HR 5.41; 95%CI 1.80-16.28; P=0.003]. This association remained significant when including Age and grade III-IV GVHD in multivariate analysis: RPCMV [HR 7.57; 95%CI 2.20-25.9;P=0.0013=], Age [HR 1.03; 95% CI 0.99-1.08; P=0.16] and GVHD [HR 2.91; 95%CI 0.87-9.75; P=0.08]. RP-CMV was not a risk factor for mortality within the NPGF population [HR 0.75; 95%CI 0.29-01.97; P=0.56]. Those with RP-CMV experienced an increase in their CMV viral load within a median of 4 days range (2-86) after achievement of an undetectable result. Those with PGF and RP-CMV had an average of 1.4 extra admissions with an average length of stay of 80 days, those with S-CMV reactivation had an average of 1.6 extra admissions with an average length of stay of 35 days, and those with N-CMV had an average of 1 extra admission with an average length of stay of 17 days. Those with SN-CMV had an average of 1 extra admission with an average length of stay of 23 days. CMV reactivation and CMV therapy contributed to 13/17 (76%) total readmissions for those with RP-CMV compared to 5/13 (38%) readmissions for those with S-CMV.
Conclusion
Patients with PGF and RP-CMV are at high risk of mortality as well as more lengthy hospital admissions of which CMV reactivation and CMV therapy is a potential contributor. Targeted use of novel therapies to prevent CMV reactivation in patients with PGF may improve both bone marrow function and survival leading to less resource utilization.
Chee: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Koldej: CRISPR Therapeutics: Research Funding. Ritchie: BMS: Research Funding; Takeda: Research Funding; CRISPR Therapeutics: Research Funding; Amgen Inc: Honoraria, Research Funding; CSL: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal